As the Coronavirus pandemic trudges on, another viral illness has caught the world's consideration: mpox (previously called monkeypox). At the hour of this composition, there have been 38,019 mpox cases in 93 nations, with 37,623 of those cases happening in nations that poor person generally revealed the illness. In excess of 11,800 cases have been accounted for in the U.S. alone — more than some other country on the planet — inciting the Biden organization to proclaim mpox a general wellbeing crisis. This naturally starts the inquiry: do worldwide mpox episodes mark the start of another all out pandemic? While there are similitudes among mpox and Coronavirus (e.g., both are zoonotic illnesses), there are recognizing highlights with significant ramifications for sickness transmission and episode elements. This article sums up the similitudes and contrasts between Coronavirus and mpox, and the infections that cause them. To find out more, look at our Coronavirus and Mpox Asset pages.
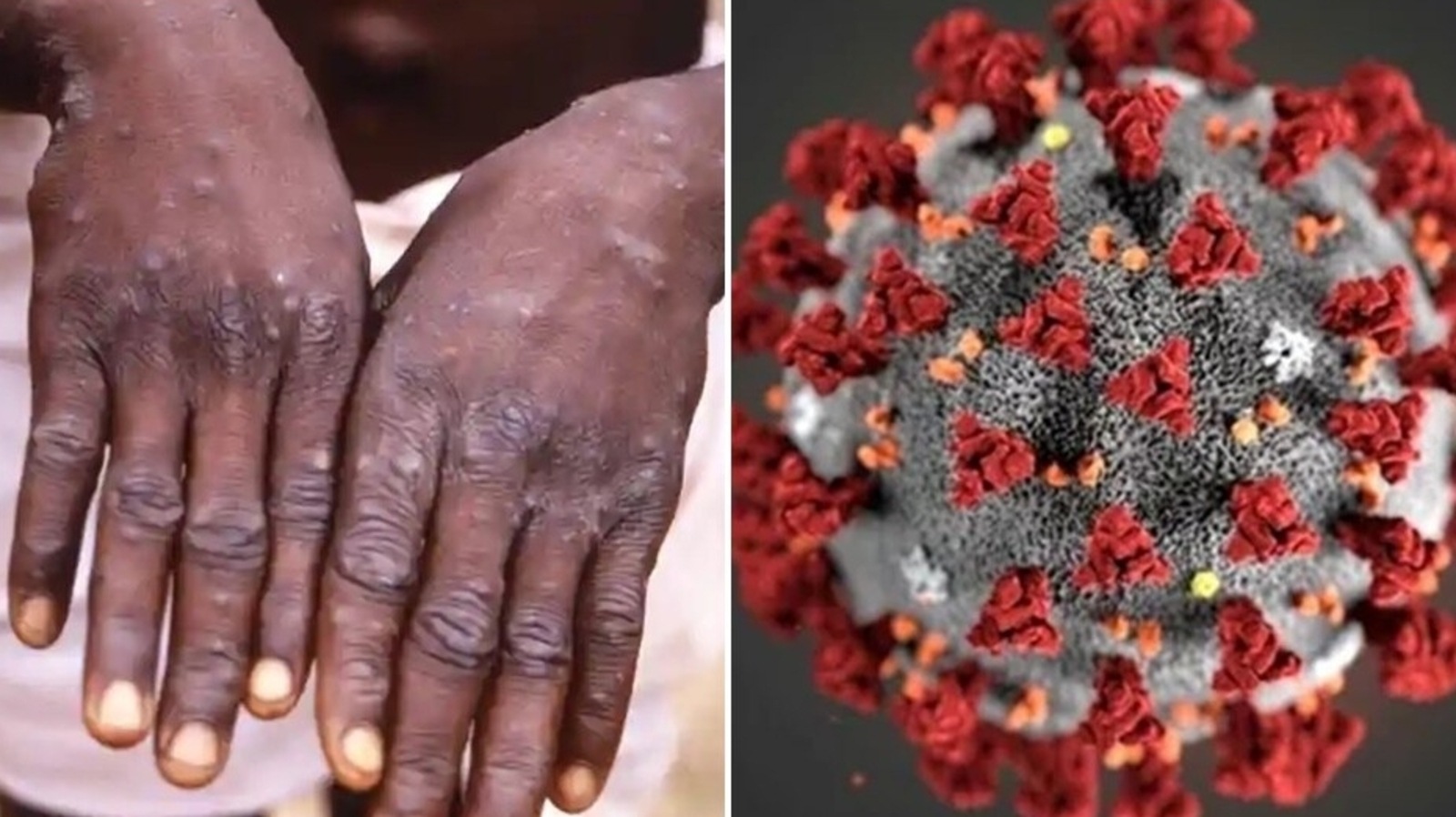
SARS-CoV-2 and Mpox Infection: Construction and Development
Construction and Genome
Primarily speaking, SARS-CoV-2 (the reason for Coronavirus) and mpox infection (MPV) are totally different. SARS-CoV-2, similar to all Covids, is an encompassed single-abandoned RNA infection. It is little (~100 nm in width), round and finished with a porcupine-like sheath of spike (S) proteins. S proteins tie to have cells by means of angiotensin-changing over compound 2 (ACE2), a protein universally communicated by organs all through the human body, to start contamination.
MPV is an individual from the Poxviridiae family — the infection is encompassed, block formed and huge (220-450 nm long). Its twofold abandoned DNA genome is exemplified in a center containing catalysts required for replication and avoidance of host resistant safeguards. Like SARS-CoV-2, MPV has surface proteins that work with its entrance into have cells. Notwithstanding, as opposed to a solitary protein, poxviruses utilize 11 to 12 transmembrane proteins to combine with have cells, probable restricting glycosaminoglycans or laminin on the cell surface.
Development and Variations
The distinctions in the genomes of SARS-CoV-2 and MPV have significant transformative repercussions. RNA infections, similar to SARS-CoV-2, can be messy replicators. RNA polymerase, which duplicates the viral genome, misses the mark on capacity to catch and fix replication blunders. Dissimilar to other RNA infections, Covids truly do have a chemical (i.e., an exoribonuclease) with some editing skill. Be that as it may, while this might slow the securing of transformations in SARS-CoV-2, it doesn't stop them out and out. Thus, arbitrary changes foster that can, if helpful for viral wellness, immediately become broad. This has been evident all through the Coronavirus pandemic. In 2021, the SARS-CoV-2 Delta variation overwhelmed the pandemic scene. When 2022 moved around, Omicron, which spreads simpler from one individual to the next, supplanted Delta as the most predominant variation. The expanded contagiousness of Omicron is attached to a large number of S protein transformations that control restricting to ACE2 and elevate the capacity to dodge have antibodies.
There are 2 known viral clades of MPV: the Congo Bowl clade and the less harmful West African clade, which underlies current episodes in non-endemic nations. DNA infections, as MPV, don't change as unreservedly as RNA infections. The proteins associated with DNA viral replication (i.e., DNA polymerase) are better at editing and fixing blunders than those in RNA viral replication (i.e., RNA polymerase). Poxviruses normally secure around 1-2 changes each year. In any case, proof proposes that MPV has procured almost 50 transformations contrasted with strains distinguished in 2018-2019. In the event that/how these hereditary changes impact the spread of mpox is muddled. What scientists can be sure of is that a large portion of the transformations bear the characteristic of a human antiviral catalyst, APOBEC3, which alters base matches in viral genomes. The changes, in this manner, don't mirror the infection's irregular transformation rate, yet appear to be characteristic of time spent in people (to be sure, information propose MPV might have been coursing among human populaces in Africa and Europe for quite a long time before the convergence of cases started in May 2022). This varies from transformation designs in SARS-CoV-2, which are to a great extent attached to replication blunders that might become fixed in a populace.
Read Also : What is the most visited destination in the United States?
As the Coronavirus pandemic trudges on, another viral illness has caught the world's consideration: mpox (previously called monkeypox). At the hour of this composition, there have been 38,019 mpox cases in 93 nations, with 37,623 of those cases happening in nations that poor person generally revealed the illness. In excess of 11,800 cases have been accounted for in the U.S. alone — more than some other country on the planet — inciting the Biden organization to proclaim mpox a general wellbeing crisis. This naturally starts the inquiry: do worldwide mpox episodes mark the start of another all out pandemic? While there are similitudes among mpox and Coronavirus (e.g., both are zoonotic illnesses), there are recognizing highlights with significant ramifications for sickness transmission and episode elements. This article sums up the similitudes and contrasts between Coronavirus and mpox, and the infections that cause them. To find out more, look at our Coronavirus and Mpox Asset pages.
SARS-CoV-2 and Mpox Infection: Construction and Development
Construction and Genome
Primarily speaking, SARS-CoV-2 (the reason for Coronavirus) and mpox infection (MPV) are totally different. SARS-CoV-2, similar to all Covids, is an encompassed single-abandoned RNA infection. It is little (~100 nm in width), round and finished with a porcupine-like sheath of spike (S) proteins. S proteins tie to have cells by means of angiotensin-changing over compound 2 (ACE2), a protein universally communicated by organs all through the human body, to start contamination.
MPV is an individual from the Poxviridiae family — the infection is encompassed, block formed and huge (220-450 nm long). Its twofold abandoned DNA genome is exemplified in a center containing catalysts required for replication and avoidance of host resistant safeguards. Like SARS-CoV-2, MPV has surface proteins that work with its entrance into have cells. Notwithstanding, as opposed to a solitary protein, poxviruses utilize 11 to 12 transmembrane proteins to combine with have cells, probable restricting glycosaminoglycans or laminin on the cell surface.
Development and Variations
The distinctions in the genomes of SARS-CoV-2 and MPV have significant transformative repercussions. RNA infections, similar to SARS-CoV-2, can be messy replicators. RNA polymerase, which duplicates the viral genome, misses the mark on capacity to catch and fix replication blunders. Dissimilar to other RNA infections, Covids truly do have a chemical (i.e., an exoribonuclease) with some editing skill. Be that as it may, while this might slow the securing of transformations in SARS-CoV-2, it doesn't stop them out and out. Thus, arbitrary changes foster that can, if helpful for viral wellness, immediately become broad. This has been evident all through the Coronavirus pandemic. In 2021, the SARS-CoV-2 Delta variation overwhelmed the pandemic scene. When 2022 moved around, Omicron, which spreads simpler from one individual to the next, supplanted Delta as the most predominant variation. The expanded contagiousness of Omicron is attached to a large number of S protein transformations that control restricting to ACE2 and elevate the capacity to dodge have antibodies.
There are 2 known viral clades of MPV: the Congo Bowl clade and the less harmful West African clade, which underlies current episodes in non-endemic nations. DNA infections, as MPV, don't change as unreservedly as RNA infections. The proteins associated with DNA viral replication (i.e., DNA polymerase) are better at editing and fixing blunders than those in RNA viral replication (i.e., RNA polymerase). Poxviruses normally secure around 1-2 changes each year. In any case, proof proposes that MPV has procured almost 50 transformations contrasted with strains distinguished in 2018-2019. In the event that/how these hereditary changes impact the spread of mpox is muddled. What scientists can be sure of is that a large portion of the transformations bear the characteristic of a human antiviral catalyst, APOBEC3, which alters base matches in viral genomes. The changes, in this manner, don't mirror the infection's irregular transformation rate, yet appear to be characteristic of time spent in people (to be sure, information propose MPV might have been coursing among human populaces in Africa and Europe for quite a long time before the convergence of cases started in May 2022). This varies from transformation designs in SARS-CoV-2, which are to a great extent attached to replication blunders that might become fixed in a populace.
Read Also : What is the most visited destination in the United States?