How Do Bacteria Control Pathogenicity With Quorum Sensing? In bacteria, quorum sensing is a well-researched intercellular communication mechanism that is activated by and dependent upon chemical signaling molecules known as autoinducers. When a colony of bacteria reaches a certain cell density, the procedure enables them to collectively control gene expression and, therefore, their behavior. By coordinating the behaviors of all members of a bacterial group, a population of bacteria may function similarly to a multicellular creature.
Numerous bacterial functions, such as bioluminescence, symbiosis, virulence gene expression, antibiotic resistance, and biofilm formation, are regulated by quorum sensing. and know more about the how do bacteria control pathogenicity with quorum sensing?
How Do Bacteria Control Pathogenicity With Quorum Sensing?
What causes quorum sensing to occur?
The buildup of signaling molecules called autoinducers in the extracellular environment initiates quorum sensing. As the bacterial population density rises, so do the concentrations of these autoinducers. Once the quantity of autoinducers exceeds a threshold, bacteria use certain receptors or sensor proteins in their cell membrane to detect the presence of these chemicals. When these molecules are found, a sequence of signaling cascades is set off, which eventually results in altered gene expression and coordinated bacterial population behaviors.
Quorum sensing's four phases
Quorum sensing generally takes place in four major phases. These include the generation and accumulation of autoinducers, the identification of autoinducers, and the control of gene expression. As the bacteria achieve and sustain the threshold concentration, they continue to manufacture autoinducers at a baseline level. Bacteria can monitor their population density and modify their activity as necessary thanks to the four phases.
Revolutionary findings in quorum sensing
The late 1960s and early 1970s saw the publication of some of the first indications that quorum sensing was occurring in bacterial communities.2 The first scientists discovered that Streptococcus pneumoniae needed to produce extracellular chemicals in order to be genetically competent. Second, it was shown that the activity of signaling molecules emitted from bacterial cells also affected the luminescence of various marine bacteria. But it wasn't until the 1980s that two important findings began to solidify the notion that bacteria could coordinate their activities and communicate according to the density of their populations.
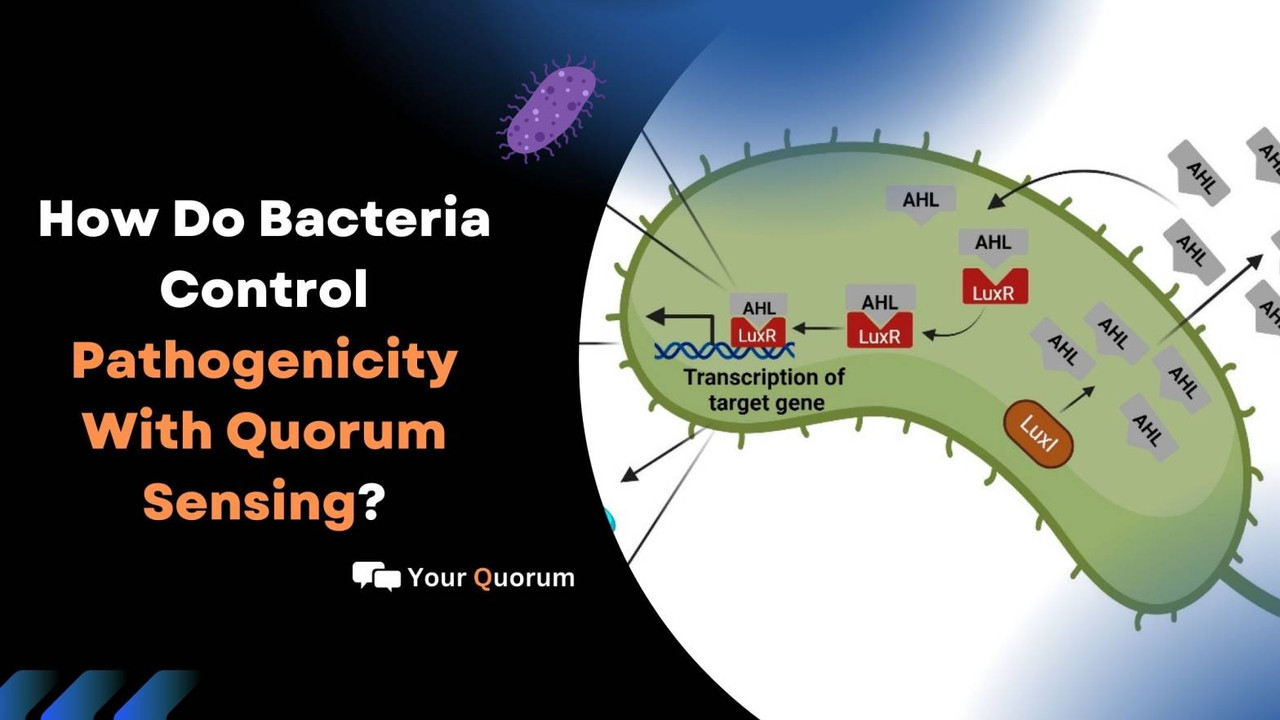
The System of LuxR-LuxL
Another advance was the first description of the LuxR-LuxL system's operation in V. fischeri. The LuxI enzyme, which produces N-3-oxo-hexanoyl-L-homoserine lactone, and the LuxR receptor protein, which senses the presence of acyl-homoserine lactone and triggers gene expression, make up the system.
Quorum sensing using Gram-positive and Gram-negative
A wide variety of signaling molecules that fall into two main categories of quorum sensing molecules have surfaced since these ground-breaking findings. Gram-positive bacteria employ quorum sensing molecules, which are either short peptides or amino acids, whereas Gram-negative bacteria use molecules associated with N-acyl homoserine lactones (Fig. 3).
Quorum sensing phenomenon examples
Virulence
The expression of many virulence factors that are necessary for bacterial pathogenicity is controlled by quorum sensing. When additional bacteria are present, it enables pathogens to control the production of biofilms or the expression of virulence factors such particular toxins (see the next section). It could be feasible to lessen virulence, lessen the severity of infections, or lessen the need for medications to control pathogenic bacteria by interfering with quorum sensing.
An excellent example of a pathogenic bacteria that generates several virulence factors that are strictly controlled by quorum sensing is Pseudomonas aeruginosa. This comprises endotoxins that prevent host cells from synthesizing proteins, proteases that aid in tissue damage and immune evasion (such as LasA, LasB, and AprA proteases), and the development of several secretion systems connected to immune response invasion and evasion. Later, we will see how another virulence factor, the blue-green pigment pyocyanin, contributes to tissue damage and how scientists are searching for potential treatments to lessen its effects.
Formation of biofilms
Biofilm production is also influenced by quorum sensing. Layers of microscopic organisms that have gathered to form a colony are called biofilms. When bacteria produce biofilms that shield them from both antimicrobial agents and immunological responses, they become resistant to antibiotics. According to the application note, biofilm production may be readily measured using crystal violet staining and detection on a microplate reader. A crystal violet biofilm quantification technique is used to test new bacteriophages for antibacterial qualities.
Applications of microplate readers in quorum sensing studies
In the biological sciences, microplate readers are indispensable instruments for a variety of applications. They enable researchers to conduct a variety of tests, investigate bacteria in a variety of methods, and explore a broad range of applications in the field of microbiology, including the hunt for novel antimicrobials. The ability to choose how rapidly bacteria may grow in various environments is essential for many of these applications. By measuring the optical density at 600 nm, or OD600 as it is often called, one may easily gauge the development of bacteria. The BMG LABTECH blog post explains how to measure bacterial growth and the underlying concepts. Measure microbial growth using OD600.
Bacterial proliferation is another crucial characteristic that must be examined while researching quorum sensing. Bacterial growth must be monitored in conjunction with quorum sensing, for instance, if researchers are looking for quorum sensing inhibitors. Instead of killing or stopping the development of bacteria, quorum sensing agents work by stopping harmful processes such as the creation of toxins, the formation of biofilms, swimming, swarming, or motility. Quorum sensing inhibitors, in other words, prevent pathogenicity instead of bacterial growth. Therefore, it is required to show that there is no effect on growth concurrent with the particular quorum sensing activity that is being studied.
Bioluminescence as a reading method
By measuring bioluminescence and bacterial growth simultaneously, the application note examined the impacts of quorum sensing in monitoring bacterial cell-to-cell communication using a BMG LABTECH microplate reader. The effect of quorum sensing on this Gram-negative bacteria was investigated by cultivating several strains of V. fischeri under various specified environmental conditions. Batch growth curves for V. fischeri strains cultivated in various mediums are shown in Figure 5. This kind of growth curve may be tracked concurrently with bioluminescence measurements or other quorum sensing outputs.
Cyclodextrins prevent the production of virulence-causing pigments.
Probably the most researched quorum sensing pathogen to date is P. aeruginosa. Quorum sensing controls around 6% of this organism's genes, which are involved in biofilm development, pigment synthesis, and pathogenicity. Researchers examined how cyclodextrins affect these pigment virulence factors, which disrupt several cellular processes during infection, in the study "Quorum quenching effect of cyclodextrins on the pyocyanin and pyoverdine production of Pseudomonas aeruginosa."
The manufacture of pigment virulence components was inhibited by both α- and β-cyclodextrin, indicating the potential use of cyclodextrins as quorum sensing inhibitors that may be employed as an antivirulence tactic.
Using an Omega series microplate reader, the fluorescence of pigment production was measured in the presence or absence of the various inhibitors to carry out the quorum sensing tests. Throughout the trials, cell growth was also tracked, and the addition of cyclodextrin inhibitors did not significantly alter it.10.
Conclusion: How Do Bacteria Control Pathogenicity With Quorum Sensing
Researchers are looking for novel approaches to take use of quorum sensing's special characteristics. For instance, they are seeking for novel options to destroy biofilms, attenuating virulence factors, and creating synthetic chemicals that imitate the natural signals of quorum sensing. Additionally, they are looking for new pharmacological targets and potentially advantageous combinations of new and current medications.
Applications in synthetic biology, microbiome regulation, ecological interaction research, and environmental monitoring are among the new fields of quorum sensing inquiry.
No comments